
Sustainability
Continuous Improvement of Responsible Care Activities
- HOME >
- Sustainability >
- Continuous Improvement of Responsible Care Activities >
- Management of Chemical Substances
Management of Chemical Substances
Policy/Philosophy
During the World Summit on Sustainable Development (WSSD) held in 2002, the "2020 goal" were agreed upon to minimize the negative impact on people's health and environment caused by the production and use of chemicals. This was an effort taken to ensure that chemicals do not cause health damage or adverse effects on the environment through safe production, transportation, use, consumption, and disposal. To achieve the "2020 goal", the "Strategic Approach to International Chemicals Management (SAICM)" for promoting risk reduction based on scientific risk assessment, collection and provision of information, and other measures were adopted at the "International Conference on Chemicals Management (ICCM)" in 2006.
At Nissan Chemical, through responsible care activity, we strive to minimize the negative impact of chemicals on people’s health and the environment during their lifecycle in line with the domestic SAICM implementation plan. For example, we have a policy which includes below.
- advancing the elimination of substances subject to the Stockholm Convention in the Company
- promoting initiatives such as alternative when new candidate substances are added to the Stockholm Convention in the future
- promoting a step-by-step reduction in manufacturing, use, sales, and procurement of substances subject to the Rotterdam Convention and the Montreal Protocol
System
Activities
Strengthening the Chemicals Management System
In order to properly manage chemicals, we have built a system to manage and provide legal and regulatory information and risks and hazards information related to the chemicals which we manufacture and use. Using safety data sheet (SDS) creation and management system, we compile a database of the chemicals on the risks and hazards information, GHS classification, etc. Then, we provide these information to customers and related parties through such as labels, SDSs, yellow cards. In addition, we ensure thorough compliance with laws and regulations by usng a legal and regulatory information service, and strive to prevent accidents by utilizing risk and hazards assessments of chemicals in accordance with the Industrial Safety and Health Act.
Going forward, we will build a more robust chemicals management system to promote autonomous management of chemical substances.
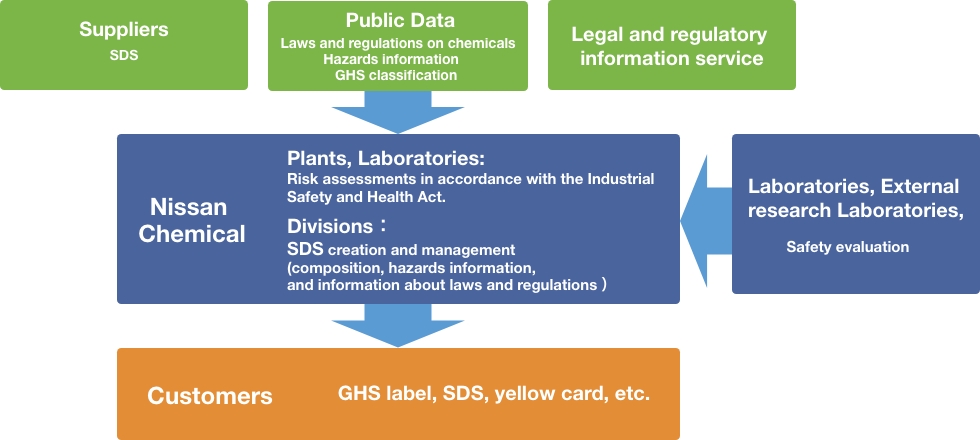
Chemicals Management System
Risk Assessment of Chemicals
We perform a risk assessment (prior assessment) of each step in handling chemicals, such as the research and development, manufacture, sales and revision. The risk assessment is performed based on legal and regulatory information, safety data evaluated by internal or external laboratories, safety data obtained from literature, and data on physicochemical properties and work environment conditions, etc. Based on the results of risk assessment, we take appropriate measures; i.e., legal and regulatory compliance, improving facilities to reduce worker exposure at manufacturing sites, improvement of operation procedures, clarification and documentation of the procedures, and the training, etc. These results are made known to all the relevant people in the Company.
Besides, we participate in Long-Range Research Initiative, an international initiative promoted by Japan Chemical Industry Association (JCIA) that seeks to provide long-term support for research on the impact of chemicals on human health and the environment. The activities we engage in aim to advance research on the assessment of risks to human health and the environment.
| Main Categories for Risk Assessment |
|---|
|
Disclosure of SDS of Products
To ensure that our chemicals are used safely by our customers, we provide SDSs of all products. Customers and users can download the SDSs for all agrochemicals from our website (https://www.nissan-agro.net/products/index.php). In addition, we have built a system that allows our employees to obtain information anytime on risks and hazards, laws and regulations, methods of transportation, storage and disposal of products from our internal SDS database.
Disclosure of GPS Safety Summary of Chemicals
As part of the GPS※1 / JIPS※2 activities for minimizing the risks posed by the chemical substances throughout the entire value chain, we compile safety information of our chemical products for which we have conducted risk assessments in GPS / JIPS Safety Summary and make them available through the JCIA website (https://www.jcia-bigdr.jp/jcia-bigdr/material/icca_company_list).
- 1 Global Product Strategy
- 2 Japan Initiative of Product Stewardship
Consideration for Animal Testing
Biological Research Laboratories has established Animal Testing Guideline of Nissan Chemical in accordance with the Three Rs principles of animal welfare (Replacement with alternative methods, Reduction in the use of laboratory animals and Refinement of methods for reducing pain), and laws and regulations such as the “Act on Welfare and Management of Animals”. Following this guideline, the Institutional Animal Care and Use Committee established in the Company ethically and scientifically examines whether or not all animal experiments conducted in Biological Research Laboratories are acceptable. We also conduct self-inspection every fiscal year to confirm that animal experiments are properly conducted in accordance with various laws and regulations and guidelines for animal testing.
Due to these initiatives, the Biological Research Laboratories has obtained third-party certification as a facility conducting animal testing appropriately from Japan Pharmaceutical Information Center.





